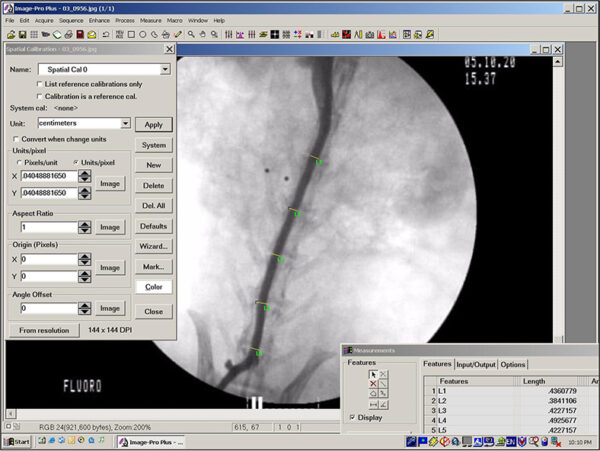
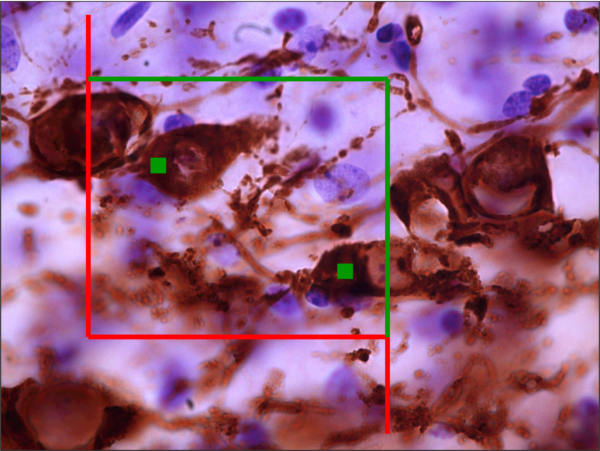
Image Analysis
Image analysis involves the computer-assisted quantification of various measurement parameters performed on digital color or black and white photographs of histologic sections. Typical measurements include object counts, linear measurements, area measurements, and relative color intensities. Image analysis has proven valuable in studies that concern the identification and documentation of subtle induced changes in tissues. Compared to the less precise or more laborious manual quantification methods it replaces (e.g. semi-quantitative grading systems, microscope stage quantification), image analysis has numerous potential advantages, including improved objectivity and consistency, enhanced sensitivity, and shorter turnaround times, especially for projects in which measurements can be automated. Image analysis also provides a permanent record of the data and of data collection, produces results that are highly amenable to statistical analysis, and allows the user to collect data that would otherwise be difficult or impossible to obtain. EPL creates software-based macro routines for each study in order to automate repetitive tasks and ensure that quantitative data are collected consistently.
Stereology is similar to image analysis, but allows for the quantitative assessment of objects in three dimensions. Examples of measurements that can be obtained include total cell count, cell density, cell size, tissue volume, and the length density and/or total length of linear structures such as nerve process or blood vessels. Current design-based stereological methods have evolved to provide results that are unbiased, efficiently obtained, and repeatable. A key stereological practice that is intended to reduce bias and aid both efficiency and repeatability has been termed systematic random sampling (SRS). According to this concept, selection of the initial tissue measurement site is randomized, but selection of ensuing measurement sites is regular. One of the most commonly used stereological tools for cell counting, especially in neuropathology, is the optical fractionator approach. This approach employs a virtual three-dimensional geometric probe called a disector that is used to count the number of objects (e.g., cells) in a well-defined, three-dimensional region (e.g., the hippocampal region of the brain). The results of disector counting are unaffected by the size, shape, or orientation of the objects. In anatomic pathology, the disector principle may be used to count cells in paired sets of thin histologic sections (physical disector) or at various focal plane depths in a subsampled series of thick histologic sections (optical disector). A disadvantage of the physical disector approach is the need to align the paired sections based on morphologic landmarks to ensure that a cell is never counted twice; however, the optical disector approach may not be feasible if thick sections cannot be prepared or if the cell type of interest cannot be reliably identified. The optical fractionator is a variation of the optical disector approach. The fractionator differs from earlier methods in that it is not necessary to determine the volume of the region in order to estimate the total cell number. This is because unlike prior methods, the entire region of interest is sampled.
In terms of obtaining counts and measurements of objects in complex three-dimensional structures, computerized stereological analysis is only the final step in an elaborate and exacting process. Considerations of tissue collection and preparation are at least equally important. That is why among laboratories performing stereological procedures it is generally considered standard practice and essential that assay optimization be conducted prior to the initiation of experiments intended to provide definitive data. The purpose of this optimization work is to refine various aspects of tissue preparation and evaluation that include but are not limited to dissection technique; tissue preservation, transport and storage; the plane of tissue sectioning and microtomy technique; staining procedures; the verification of consistent microanatomical landmarks, and the establishment of various parameters specific to stereologic cell counting. It is unlikely that repeatable results can be obtained if inadequate consideration is given to any of these aspects.
Although almost anything that can be imaged can be analyzed with these tools, optimal results are achieved when studies are designed as morphometric projects from the ground up. Consistent and good quality sampling is essential, from the necropsy phase through the actual quantitative assessment. By using EPL for your image analysis or stereology needs, you will receive the most accurate data for interpretation.
Examples of image analysis projects:
Areas of tissue reaction to implanted devices
Comparative intestinal wall thickness (villus heights, crypts depths)
Determination of tumor (or granuloma) sizes and numbers
Differential counts of germinal cell types in gonad sections
Immunohistochemical labeling for cell proliferation or cell death
Linear measurements for developmental neurotoxicity studies
Quantification of electrophoretic gel intensities
Thyroid gland follicle sizes during development or endocrine modulation
Ultrastructural studies of peripheral nerve demyelination
Whole-body growth studies
Examples of stereology projects:
Quantification of neurons in certain brain regions
Counts of nerve cell bodies in ganglia
Nerve ending density in various tissues
Differential germ cell counts (entire gonad)
Advantages of using EPL for your Stereological projects:
EPL technicians have extensive expertise in the creation of thick frozen or paraffin-embedded sections for cell counting purposes
Immunohistochemical staining and evaluation can be performed using either chromogenic (diaminobenzidine, DAB) or fluorescent markers
Stereology projects supervised by an ACVP board-certified pathologist
EPL uses MBF Bioscience’s popular and robust StereoInvestigator software
Learn More
Fill out the form below or call (703) 471-7060 for more information regarding our imaging and whole slide scanning services.